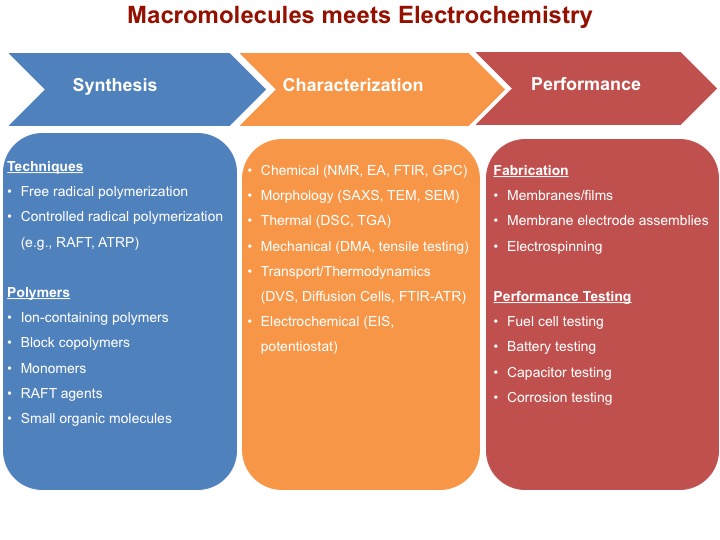
Our laboratory combines macromolecules (polymers) with electrochemistry to design fuel cells, batteries, capacitors, and corrosion-resistant coatings, to name just a few applications. We synthesize new polymers for the advancement of these applications and transport and thermodynamics of ions and small molecules in polymers guide our synthetic design principles.
Funded Research Projects
1. Polymerized Ionic Liquids: Alkaline Fuel Cells and Lithium Ion Batteries
Solid-state polymer electrolytes with high ionic conductivity have been the subject of extensive research for electrochemical devices, such as fuel cells and batteries. In our laboratory, we are synthesizing and characterizing a new class of solid-state polymer electrolyte: polymerized ionic liquid block copolymers (PILBCPs), which combine the benefits of block copolymers and ionic liquid-based chemistry. We are exploring the transport of hydroxide and lithium in this new class of materials for application to alkaline fuel cells and lithium ion batteries.
For alkaline fuel cells (AFCs), chemically stable, mechanically robust, highly hydroxide conductive anion exchange membranes (AEMs) are required as electrolytes to realize high power density, long-lasting AFCs. AFCs are attractive replacements to traditional proton exchange membrane fuel cells, because they do not require the use of expensive precious metal catalysts, but can operate with cheaper non-noble metal catalysts (e.g., Ni) due to their facile electrokinetics in alkaline conditions. Our new PILBCP AEM demonstrates that chemically stable, mechanically robust, highly hydroxide conductive AEMs with new cation chemistries are now possible.
Surprisingly, the hydroxide conductivity of our PILBCP AEM is not only higher (over an order of magnitude) than its random copolymer analog at the same ion and water content, but also higher than its homopolymer analog, which has a higher ion and water content than the block copolymer. This is a product of PILBCP’s nanostructured morphology, where nanochannels accelerate hydroxide ion mobility, which should subsequently result in higher power densities in an AFC. These results should have a significant impact on low-cost (platinum-free) long-lasting solid-state AFCs. We are now exploring morphology-transport relationships in PILBCP AEMs under controlled environments (humidity, temperature, voltage) using in situ time-resolved infrared spectroscopy and in situ multi-angle X-ray scattering techniques. This will guide the future synthesis of different PILBCP AEMs with optimal morphology and ion transport. We are also integrating our PILBCP AEMs into AFCs and testing fuel cell performance.
We have also developed lithium-ion conducting PILBCP solid-state polymer electrolytes (SPEs) for integration into lithium-ion batteries. Currently, high manufacturing costs and concerns about battery safety and stability hinder the market growth of the lithium-ion battery. Replacing liquid-based electrolytes in lithium-ion batteries with SPEs can alleviate many cost, safety, and stability concerns and is arguably the most attractive new technology for rechargeable electric power sources. However, this will require SPEs with properties of high lithium-ion conductivity, excellent mechanical properties, and good processability. Our recent results demonstrate robust and processable PILBCP SPEs with high lithium-ion conductivity. These results are a product the nanostructured morphology of our PILBCP, where nanochannels accelerate the lithium-ion transport, which should result in faster charge/discharge rates in batteries. The PIL chemistry in PILBCP also allows for a broad electrochemical window and is non-flammable and non-volatile. The chemistry of these nanochannels (PIL) and nanostructured morphology in our PILBCP are both novel and commercially viable to batteries. We are now exploring transport-morphology-processing relationships to guide the synthesis of new PILBCPs and integrating PILBCP SPEs into coin cells for battery testing.
Current Funding:
National Science Foundation, Award Number: CBET-1703645
National Science Foundation, Award Number: CBET-170621
Army Research Office, Award Number: W911NF-14-1-0310
Kraton Polymers
Selected Publications and Patents:
Chen, T. L.; Lathrop, P. M.; Sun, R.; Elabd, Y. A. Lithium-Ion Transport in Poly (ionic liquid) Diblock Copolymer Electrolytes: Impact of Salt Concentration and Cation and Anion Chemistry. Macromolecules, 2021, 54, 8780-8797. DOI: 10.1021/acs.macromol.1c00694
Hwang, M.; Sun, R.; Willis, C.; Elabd, Y. A. Solid‐State Alkaline Fuel Cell Performance of Pentablock Terpolymer with Methylpyrrolidinium Cations as Anion Exchange Membrane and Ionomer. Fuel Cells, 2020, 20, 624-633. DOI: 10.1002/fuce.202000092
Sun, R.; Elabd, Y. A. Synthesis and High Alkaline Chemical Stability of Polyionic Liquids with Methylpyrrolidinium, Methylpiperidinium, Methylazepanium, Methylazocanium, and Methylazonanium Cations. ACS Macro Lett. 2019, 8, 540-545. DOI: 10.1021/acsmacrolett.9b00039
Meek, K.M.; Sun, R.; Willis, C.; Elabd, Y.A. Hydroxide Conducting Polymerized Ionic Liquid Pentablock Terpolymer Anion Exchange Membranes with Methylpyrrolidinium Cations. Polymer 2018, 134, 221-226. DOI: 10.1016/j.polymer.2017.11.050
Elabd, Y.A.; Winey, K.I.; Ye, Y.; Choi, J.-H. Sharick, T.-S. S. Polymerized Ionic Liquid Block Copolymers As Battery Membrane. U.S. Patent 9,365,688, June 14, 2016.
Nykaza, J.R.; Benjamin, R.; Meek, K.M. Elabd, Y.A. Polymerized Ionic Liquid Diblock Copolymer as an Ionomer and Anion Exchange Membrane for Alkaline Fuel Cells. Chemical Engineering Science 2016, 154, 119-127. Invited Contribution, Special Issue on Recent Advances in Energy Conversion and Storage Devices DOI: 10.1016/j.ces.2016.05.041
Meek, K.M.; Nykaza, J.R.; Elabd, Y.A. Alkaline Chemical Stability and Ion Transport in Polymerized Ionic Liquids with Various Backbones and Cations. Macromolecules 2016, 49, 3382-2294. DOI: 10.1021/acs.macromol.6b00434
Meek, K.M.; Elabd, Y.A. Polymerized Ionic Liquid Block Copolymers for Electrochemical Energy. Journal of Materials Chemistry A 2015, 3, 24187-24194. Invited Contribution DOI: 10.1039/C5TA07170D
Meek, K.M.; Elabd, Y.A. Alkaline Chemical Stability of Polymerized Ionic Liquids with Different Cations. Macromolecules 2015, 48, 7071-7084. DOI: 10.1021/acs.macromol.5b01223
Meek, K.M.; Sharick, S.; Ye, Y.; Winey, K.I.; Elabd, Y.A. Bromide and Hydroxide Conductivity-Morphology Relationships in Polymerized Ionic Liquid Block Copolymers. Macromolecules 2015, 48, 4850-4862. DOI: 10.1021/acs.macromol.5b00926
2. Super Conducting Nanofibers: Ultra-Low Platinum Electrodes for Proton Exchange Membrane Fuel Cells
Nafion is the most frequently used polymer in proton exchange membrane fuel cells (PEMFCs), actuators, and a variety of sensors. Recently, we reported the high proton conductivity of a single high purity Nafion nanofiber (1.5 S/cm), which is an order of magnitude higher than the bulk Nafion film (~ 0.1 S/cm). We observed a nanosize effect, where proton conductivity increases sharply with decreasing fiber diameter. X-ray scattering results confirmed an oriented ionic morphology in the nanofiber in contrast to the isotropic morphology in bulk films. We also first demonstrated the successful fabrication of high purity Nafion nanofibers (~99.9 wt%) via electrospinning and higher humidity sensitivity for nanofibers compared to the bulk. Based on these promising results, we are now exploring the impact of super conductive nanofibers in membranes, electrodes, actuators, and sensors.
One project in specific entails exploring the effect of conductive nanofibers in fuel cell electrodes. We recently reported the natural formation of conductive nanofibers in fuel cell electrodes and their impact on improving fuel cell performance and reducing the required amount of catalyst. Now, we have developed a new technique for producing robust nanofiber-based fuel cell electrodes. The motivation is to produce fuel cells with high power densities at low platinum (Pt) loadings. Currently, high cost due to the required precious metal Pt electrodes is now the major factor that has limited the mass commercialization of fuel cell vehicles, where Pt contributes to over 30% of the fuel cell engine cost. Our recent results with our new nanofiber-based fuel cell electrodes demonstrate high power densities with ultra-low Pt loadings. In addition, our new process for producing nanofiber-based electrodes provides a wide parameter space for exploration of fuel cell performance as a function of nanofiber content, size, and type. We are exploring these polymer parameters in relation to electrochemical properties and fuel cell performance.
Current Funding:
National Science Foundation, Award Number: CMMI-1661822
Selected Publications and Patents:
Elabd, Y.A.; Richey, F.W.; Wujcik, K.H. Ion Conducting Nanofiber Fuel Cell Electrodes. U.S. Patent 16,878,298, November 5, 2020.
Hwang, M.; Karenson, M. O.; Elabd, Y. A. High Production Rate of High Purity, High Fidelity Nafion Nanofibers via Needleless Electrospinning. ACS Appl. Polym. Mater. 2019, 1, 2731-2740. DOI: 10.1021/acsapm.9b00681
Hwang, M.; Elabd, Y.A. Impact of Ionomer Resistance in Nanofiber-Nanoparticle Electrodes for Ultra-Low Platinum Fuel Cells, Int. J. Hydrog. Energy 2019, 44, 6245-6256. DOI: 10.1016/j.ijhydene.2019.01.083.
Wang, X.; Richey, F.W.; Wujcik, K.; Ventura, R.; Mattson, K.; Elabd, Y.A. Effect of Polytetrafluoroethylene on Ultra-Low Platinum Loaded Electrospun/Electrosprayed Electrodes in Proton Exchange Membrane Fuel Cells. Electrochimica Acta 2014, 139, 217-224. DOI:10.1016/j.electacta.2014.06.139
Wang, X.; Richey, F.W.; Wujcik, K.; Elabd, Y.A. Ultra-Low Platinum Loadings in Proton Exchange Membrane Fuel Cell Electrodes Fabricated via Simultaneous Electrospinning/Electrospraying Method. J. Power Sources 2014, 264, 42-48. DOI:10.1016/j.jpowsour.2014.04.052
Dong, B.; Gwee, L.; Salas-de la Cruz, D.; Winey, K.I. Elabd, Y.A. Super Proton Conductive High Purity Nafion Nanofibers. Nano Letters 2010, 10, 3785-3790. DOI: 10.1021/nl102581w
Snyder, J.D.; Elabd, Y.A. Nafion® Nanofibers and Their Effect on Polymer Electrolyte Membrane Fuel Cell Performance. J. Power Sources 2009, 186, 385-392. DOI:10.1016/j.jpowsour.2008.10.039
Chen, H.; Snyder, J.D.; Elabd, Y.A. Electrospinning and Solution Behavior of Nafion and Poly(acrylic acid). Macromolecules 2008, 41, 128-135. DOI: 10.1021/ma070893g
3. In Operando Infrared Spectroelectrochemistry: Capacitors, Water Purification Membranes, and Corrosion-Resistant Coatings
In our laboratory, we have developed a new in operando infrared spectroelectrochemical technique that measures water and ion dynamics in fully integrated systems in real time (e.g., polymer/electrode composites, such as capacitors). This new technique probes ion dynamics on both a molecular and electrochemical level simultaneously in the electrode of polymer/electrode devices. These new measurements provide new insights and a fundamental understanding of water and ion dynamics in capacitors, water purification membranes, and corrosion-resistant coatings, where previously reported electrical measurements only infer mechanisms for ion migration and adsorption. This new information is guiding our design of more efficient capacitors, water purification membranes, and corrosion-resistant coatings.
Current Funding:
Army Research Laboratory, Award Number: W911NF-13-2-0046
Axalta Coating Systems
Selected Publications:
Santos, M.C.; Bendiksen, B.; Elabd Y.A. Diffusion of Liquid Water in Free-Standing Polymer Films using Pressure-Contact Time-Resolved Fourier Transform Infrared Attenuated Total Reflectance Spectroscopy. Eng. Chem. Res. 2017, 56, 3464–3476. DOI: 10.1021/acs.iecr.7b00114
Chen, T.-L.; Elabd, Y.A.; Hybrid-Capacitors with Polyaniline/Carbon Electrodes Fabricated via Simultaneous Electrospinning/Electrospraying. Electrochimica Acta 2017, 229, 65-72. DOI: 10.1016/j.electacta.2017.01.140
Santos, M.; Jing, Y.; Fang, L.; Chaplin, B.P; Elabd Y.A. Highly Porous Ti4O7 Reactive Electrode Water Filtration Membranes via Simultaneous Electrospinning/Electrospraying Method. AIChE J. 2016, 62, 508-524. Invited Contribution, Special Issue on Advances in Materials
Richey, F.W.; Tran, C.; Kalra, V.; Elabd, Y.A. Ionic Liquid Dynamics in Nanoporous Carbon Nanofibers in Supercapacitors Measured with in operando Infrared Spectroelectrochemistry. J. Phys. Chem. C 2014, 118, 21846-21855. DOI: 10.1021/jp506903m
Richey, F.W.; Dyatkin, B.; Gogotsi, Y.; Elabd, Y.A. Ion Dynamics in Porous Carbon Electrodes in Supercapacitors using in situ Infrared Spectroelectrochemistry. J. Amer. Chem. Soc. 2013, 135, 12818-12826. DOI: 10.1021/ja406120e
4. Water in Glassy Polymers: Sustainable Plastics and Biomedical Devices
Water in glassy polymers impacts our everyday lives (e.g., water bottles). We are investigating the non-equilibrium phenomena of water in glassy polymers. A fundamental understanding of sorption and diffusion of water in glassy polymers is important for the development of future materials. We have combined a number of experimental techniques, such as dynamic vapor sorption, dilation, and infrared spectroscopy, to measure changes in mass, volume and concentration simultaneously. Additionally, we have developed thermodynamic models based on a non-equilibrium equation of state approach to predict the sorption and diffusion of water in glassy polymers. We recently demonstrated the ability to predict water sorption in a number of glassy polymers at a variety of water vapor activities and temperatures using a non-equilibrium thermodynamic modeling approach: non-equilibrium statistical associating fluids theory (NE-SAFT). Additionally, we are using in situ real-time infrared spectroscopy to measure the clustering behavior of water in glassy polymers and developing a non-equilibrium thermodynamic approach to predict the diffusivity of water in glassy polymers. The results from this work are impacting the development of biodegradable renewable plastics as replacements for commodity plastics and conformal coatings on implantable biomedical devices.
Selected Publications:
Davis, E.M.; Elabd, Y.A. Water Clustering in Glassy Polymers. J. Phys. Chem. B 2013, 117, 10629-10640. DOI: 10.1021/jp405388d
Davis, E.M.; Elabd Y.A. Prediction of Water Solubility in Glassy Polymers Using Nonequilibrium Thermodynamics. Ind. Eng. Chem. Res. 2013, 52, 12865-12875. DOI: 10.1021/ie401713h
Davis, E.M.; Minelli, M.; Baschetti, M.G.; Jr.; Elabd, Y.A. Non-Fickian Diffusion of Water in Polylactide. Ind. Eng. Chem. Res. 2013, 52, 8664-8673.
Davis, E.M.; Minelli, M.; Baschetti, M.G.; Jr.; Sarti, G.C.; Elabd, Y.A. Nonequilibrium Sorption of Water in Polylactide. Macromolecules, 2012, 45, 7486-7494. DOI: 10.1021/ie302342m
Davis, E.M.; Theryo, G.; Hillmyer, M.A.; Cairncross, R.A.; Elabd, Y.A. Liquid Water Transport in Polylactide Homo and Graft Copolymers. ACS Applied Materials & Interfaces 2011, 3 (10), 3997-4006. DOI: 10.1021/am2008618
Davis, E.M.; Benetatos, N.M.; Regnault, W.F.; Winey, K.I.; Elabd, Y.A. The Influence of Thermal History on the Structure and Water Transport in Parylene C Coatings. Polymer 2011, 52 (23), 5378-5386. doi:10.1016/j.polymer.2011.08.010